Intravital Imaging
Immunohistochemistry of fixed and processed tissue specimens has provided detailed insights into various aspects of cardiovascular structure, function and diseases such as atherosclerosis. However, this technique comes at the expense of the dissection of dynamic processes in vivo. To gain insight into cardiovascular structure and function under physiologically relevant circumstances, techniques are required that enable studying processes and structures directly at the site of occurrence (i.e. in the intact living specimen).
The intravital imaging facility at the Institute for Cardiovascular Prevention (IPEK) utilizes advanced optical fluorescence techniques including dual channel fluorescence intravital microscopy (IVM) and two-photon laser scanning microscopy (TPLSM) for (molecular) imaging of cardiovascular structures and dynamic processes in intact (cardio)vascular tissues in vivo or ex vivo [1-4].
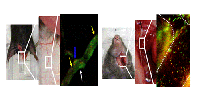
Intrinsic properties of intravital imaging modalities available at IPEK.
Classical fluorescence intravital microscopy is the model of choice for studying cell adhesion and recruitment in micro- and macrocirculation. In fact, the cell recruitment cascade was primarily identified and refined by use of brightfield or fluorescence intravital microscopy. The IPEK makes use of an upright microscope (Olympus) equipped with dual view optics (Photometrics) and a highly sensitive EMCCD camera (Hamamatsu). The latter system allows simultaneous studying of two fluorescently labeled cell subsets, chemicals, or structures. Due to its intrinsic properties (no optical sectioning, relatively large field of view, high senstivity) it is the optimal imaging method for quantification of the cell recruitment in the micro- (cremaster, mesentery, lung) and macrocirculation (carotid arteries).
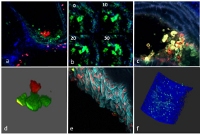
Examples of fluorescence intravital microscopy in mouse tissue.
Two-photon laser scanning microscopy (TPLSM) is optimized for more detailed studying of processes in up to four dimensions in the cardio vasculature such as cell diapedesis, cell-cell interactions, or the structure and function of materials especially deep inside large arteries or organs. In the past years, the in vivo imaging of atherosclerosis, classically limited due to movement artifacts, could be circumvented by usage of TPLSM imaging triggered on the heart and respiration cycle [6]. The latter TPLSM methodologies have been successfully applied in various studies conducted within IPEK and its collaborators [7-11].
A state-of-the-art TPLSM system (Leica SP5 MP, funded by DFG/LMU) as is available in our imaging facility, has an ideal layout for in vivo imaging of strongly motional samples, offering fast image acquisition rates (30Hz at full resolution), a large field of view (up to 700µm, dependent on objective), and highest sensitivity due to the four Hybrid detectors. Combined with image acquisition triggered on the heart- and respiration cycle of the subject, and a high speed galvanometer based z-stage (up to 500µm of travel at 40Hz) matching the scan speeds of the resonance scan head, this TPLSM system enables tracking of structures and processes in vivo over time in three dimensions. As a result, in vivo imaging of cardio vasculature can be taken to a higher level with regard to both spatial and time resolution in up to four dimensions. The group of Dr. Remco Megens will further develop and optimize in vivo TPLSM applications for imaging in (diseased) cardiovascular targets and apply them in projects studying inflammatory cell recruitment, proteases (activity), tissue structures and their function, cell-cell interactions, and cellular processes such as formation of neutrophil extracellular traps (NETs). Besides in vivo applications, TPLSM can also be applied for imaging of isolated whole mount specimen ex vivo such as various organs, microcirculatory vessel beds, lymph nodes, skeletal muscle, and large arteries.
The intravital imaging core facility at IPEK functions as a core facility for TPLSM imaging for both internal and external collaborators. Both expertise and tools are present to positively support (imaging) projects in the field of cardiovascular research and beyond.
Literature
1. Megens, R.T., et al., Imaging collagen in intact viable healthy and atherosclerotic arteries using fluorescently labeled CNA35 and two-photon laser scanning microscopy. Molecular imaging, 2007. 6(4): p. 247-60.
2. Megens, R.T., et al., Two-photon microscopy on vital carotid arteries: imaging the relationship between collagen and inflammatory cells in atherosclerotic plaques. J Biomed Opt, 2008. 13(4): p. 044022.
3. Megens, R.T., et al., Two-photon microscopy of vital murine elastic and muscular arteries. Combined structural and functional imaging with subcellular resolution. J Vasc Res, 2007. 44(2): p. 87-98.
4. Grommes, J., et al., Disruption of Platelet-derived Chemokine Heteromers Prevents Neutrophil Extravasation in Acute Lung Injury. Am J Respir Crit Care Med, 2012.
5. Megens, R.T.A., et al., Intravital imaging of phagocyte recruitment. Thrombosis and haemostasis, 2011. 105(5): p. 802-10.
6. Megens, R.T., et al., In vivo high-resolution structural imaging of large arteries in small rodents using two-photon laser scanning microscopy. J Biomed Opt, 2010. 15(1): p. 011108.
7. Drechsler, M., et al., Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation, 2010. 122(18): p. 1837-45.
8. Megens R.T.A., et al., Presence of luminal neutrophil extracellular traps in atherosclerosis. Thrombosis and Haemostasis, 2012. In press.
9. Weber, C., et al., CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest, 2011. 121(7): p. 2898-910.
10. Soehnlein, O., et al., Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Science translational medicine, 2011. 3(103): p. 103ra98.
11. Zhou, Z., et al., Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell metabolism, 2011. 13(5): p. 592-600.