Cell Migration and Clinical implications
Christian Ries
Migrating cells such as keratinocytes, fibroblasts, monocytes and mesenchymal stem cells, play key roles in multiple normal and pathological processes including wound healing, inflammation and cancer. Our research aims to gain deeper insights into molecular mechanisms that regulate the behavior and function of these cells. This may provide innovative approaches for target-directed therapeutical intervention.
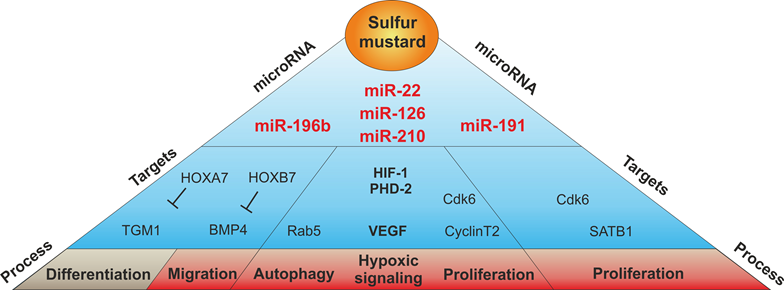
Graphical representation of a selection of sulfur mustard-regulated miRNAs with regard to their validated or potential target mRNAs and the cellular processes that might be influenced by them.
